Eli Lilly's Q2 Revenue Exceeded Expectations, New Products Coming Soon
Eli Lilly's Q2 revenue grew 36% year-on-year, exceeding expectations. The company also plans to launch more new products and expand sales of existing products in the second half of the year.
Recently, Eli Lilly and Novo Nordisk, the two "heroes" in the field of weight loss drugs in the US stock market, released their financial reports. As high-profile fields outside of artificial intelligence, the performance of these two companies has attracted much attention. After Novo Nordisk suffered a setback, Eli Lilly made a strong comeback and demonstrated its huge potential in the weight loss drug market.
Eli Lilly reported second-quarter revenue of $11.3 billion, exceeding expectations of $9.98 billion, a year-on-year increase of 36%; earnings per share soared 86% year-on-year. The company raised its full-year revenue guidance to $45.4 billion to $46.6 billion (previously $42.4 billion to $43.6 billion); adjusted earnings per share expectations were raised to $16.10 to $16.60 (previously expected to be $13.70).
Growth is expected to accelerate in the second half of the year, mainly due to the strong performance of weight loss drugs Mounjaro and Zepbound. Due to strong demand, the price of weight loss drugs is expected to increase; other drugs will maintain the same pricing as in the first half of the year, and no promotional activities are planned. In addition, the company also plans to launch more new products in the second half of the year and expand the sales of existing products.
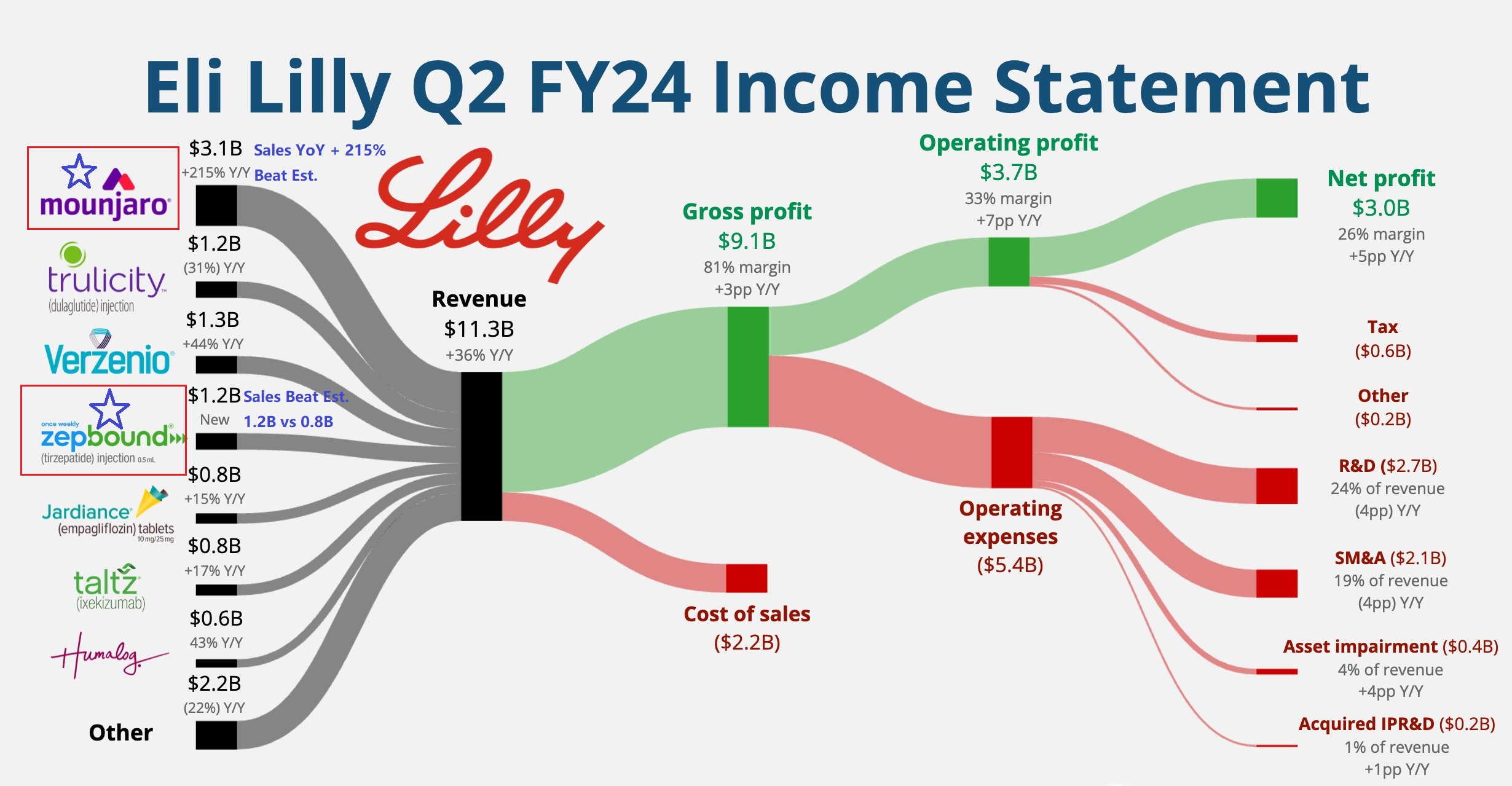
During the earnings call, Lilly executives noted:
- Will increase investment in the expansion of Zepbound and Mounjaro production capacity. Since 2020, the company has invested more than $18 billion in expanding production facilities in the United States and Europe, with positive results. Following Novo Nordisk's acquisition of Eli Lilly's main supplier Catalent in February this year, Eli Lilly is focusing on building its own production facilities.
- Plans are underway to launch 2.5 mg and 5 mg single-dose bottles of Zepbound in the coming weeks to further expand the patient base. As of July 1, more than 50% of U.S. employers provide health insurance coverage for anti-obesity drugs, and this proportion is growing to benefit more patients.
- Patients using Eli Lilly's weight loss drugs have gained the benefits of weight loss and other disease treatments, leading patients to rely on these drugs and encouraging them to complete the entire course of treatment.

In addition to weight loss drugs, Eli Lilly has also made progress in other drug areas:
- In July, the FDA approved Eli Lilly's Kisunla to treat adult patients with early-stage Alzheimer's disease (AD). Some patients have already started using this drug for clinical treatment.
- Eli Lilly's tirzepatide met all primary and key secondary endpoints in a Phase 3 clinical trial to treat moderate to severe obstructive sleep apnea and obesity; the drug also showed potential in treating heart failure. The FDA is expected to review the drug by the end of 2024. Research into the benefits of tirzepatide in reducing absenteeism and increasing workplace productivity has attracted widespread attention.
- There are currently 11 new obesity drugs in clinical trials covering various indications; Lilly has also made significant progress in oncology and neurology.
Disclaimer: The views in this article are from the original Creator and do not represent the views or position of Hawk Insight. The content of the article is for reference, communication and learning only, and does not constitute investment advice. If it involves copyright issues, please contact us for deletion.